Summary
To date, there are many ways to obtain carbon nanotubes (CNT). In this case, from the point of view, the growth mechanism of CNTs methods for their synthesis can be divided into two types - evaporation and catalytic methods. In the first case of CNTs are formed by evaporation under the action of an electric arc or a laser, a carbon sample of clusters which are aggregated into a carrier gas flow in the CNT. In the second case, the CNTs grow at the expense of carbon, which is formed by the catalytic pyrolysis of carbon-containing gas mixture, and dissolved in poatomno catalyst particles, followed by nucleation of graphite poklasternoy fragments at the interface and aggregating them into CNTs. From the point of view of chemical engineering of nanomaterials, are the most important thermo-chemical methods for the synthesis of CNTs. In addition, the characteristics of the process of catalytic pyrolysis can not just get the CNTs for further applications, and directly in the manufacturing processes to synthesize materials based on carbon nanotubes, in connection with the study of processes of synthesis of CNTs attached to their use in a variety of new materials is the actual scientific problem.The growth of carbon nanotubes can be produced as a variety of surfaces and within materials that can lead, in turn, to changes in properties such as surface and material in general. To synthesize CNTs in the materials they need to have in the structure of the catalyst particles, which should be provided with transport carbon-containing gas mixture.
Objectives
A. Material based on nanotubes and zeolite.
On the left - Photo of the zeolite after the (black) and to modify carbon nanotubes. Right - carbon nanotubes inside the zeolite material.
Studies of porosity analysis by nitrogen adsorption isotherms showed that the specific volume of adsorbed nitrogen decreased almost twice, which indicates that a substantial part has been filled with carbon nanotubes, and the remaining pores have a diameter smaller than the kinetic diameter of nitrogen molecule.
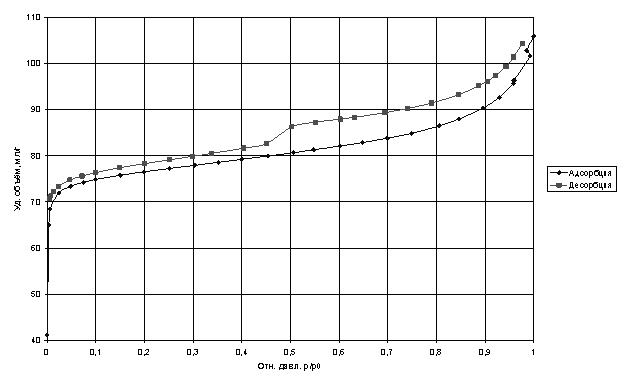
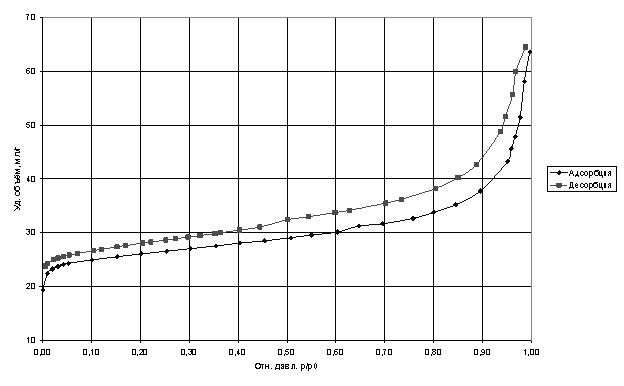
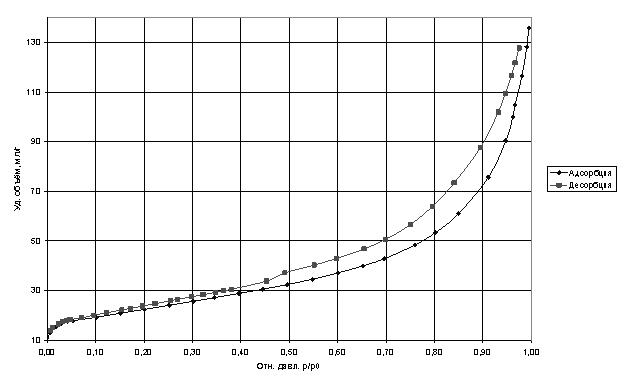
Adsorption isotherms of nitrogen at 77 K.
For comparison, studies were conducted of the sorption capacity of nitrogen molecules with carbon nanotubes, and the specific volume of the adsorbate was larger than that of the selected zeolite Saen brand for use in cryogenic pumps.
The curves were analyzed by standard adsorption models BET and BJH [Gregg SJ, Sing KSW Adsorption, Surface Area and Porosity. Academic press. In 1982. 303 p.], The main characteristics of the analysis are shown in Table 1. At the same time failed to accurately estimate the pore diameter due to the large divergence in the models in the confidence interval of at least 0.9.
The specific characteristics of the materials:
|
|
Source Zeolite CNT |
Zeolite + CNTs |
CNTs |
|
Specific surface area ь, m2/g |
259,24 |
92,38 |
78,49 |
|
Saturation capacity, mL/g |
105,99 |
64,54 |
135,9 |
2. Synthesis of carbon nanotubes in ceramic materials
To create composites based on ceramic and carbon nanotubes have been developed two methods: the synthesis of carbon nanotubes in ceramic and ceramic-based synthesis of carbon nanotubes.
The first method consists in the fact that the finished ceramic is impregnated with a catalyst which then grow inside the carbon nanotubes. The second method is to add carbon nanotubes during the synthesis of ceramics.
The figure shows photographs of the submissions received. On the left is shown the composite obtained by the synthesis of ceramics based on carbon nanotubes, on the right - without the ceramic-walled carbon nanotubes grown by standard methods.

Quartz ceramics. Left - composite ceramics obtained by the synthesis of carbon nanotubes on the right - without the ceramic-walled carbon nanotubes grown by standard methods.
As you can see, with equal amounts of matter are obtained by very different amounts of material. Measurements of the mass showed 150% weight gain, despite the fact that the nanotubes were added tens of micrograms, which amounts to hundredths of a percent in the composite. This increase is explained by the fact that carbon nanotubes having high specific surface area, lowers the free energy of formation of ceramics on the surface. Thus, they are the catalyst for the growth of pottery, showing amazing results. It is tempting outlines the prospects for using nanotubes in the ceramic industry, which will save energy on the mixing and homogenization of the ceramic mixtures.
Also, we measured the hardness of the ceramic composites.
Ceramics by Vickers hardness
|
Ceramics |
Hardness according to Vikkers |
|
without CNT |
300 MPa |
|
Ceramics grown on CNT basis |
1 GPa |
|
Ceramics with grown CNT |
200 MPa |
A significant increase in the hardness of ceramic-based CNT grown due to the denser structure of the original ceramic grains, which was created by a matrix of carbon nanotubes. Reducing the hardness of ceramic-grown carbon nanotubes, even when compared with the original composites can be explained by high dispersion of the structure, so that could not be clearly and accurately measure the composite itself.
3. Synthesis of carbon nanotubes in anodic alumina
Unfortunately, the dense "forest" of carbon nanotubes is a homogeneous emitting surface only at relatively small distances from the ends of the nanotubes, and the distance from the array is essentially a single flat emitter due to the mutual screening of neighboring carbon nanotubes. Therefore to avoid the screening wall carbon nanotubes need to grow away from each other, and therefore directed the synthesis of remotely separated nanotubes is an urgent scientific task. In addition, aiming to obtain carbon nanotubes due to a number of technological difficulties, such as the introduction of the guide field or precision control of speed and uniformity of the flow of gas mixture during the growth of CNTs. It should be noted that the receipt of an array of spaced vertical carbon nanotubes is also important to create a new type of membrane - active nanomembranes, in particular, NEMS-membranes, each of which contains two independent nanopore electrode (in the case of NEMS-membrane electrodes, one is able to controllably alter its mechanical condition .)
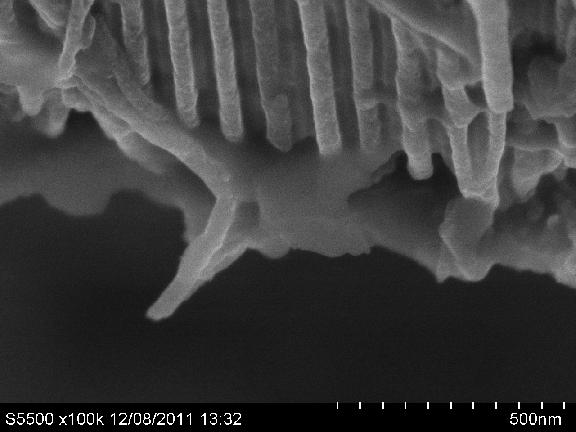
The resulting material membranes based on porous anodic aluminum oxide (PAOA) and carbon nanotubes are an array of carbon nanotubes detached from each other by isolated cell walls PAOA. SEM image of a well indicates that the diameter of carbon nanotubes strongly coincides with the pore diameter, in which they are completely filled with them.
However, the pores of aluminum oxide have here the length of the order of 10 microns. To date, current research is the technology of synthesis of carbon nanotubes in extremely thick anodic oxides with thickness of 0.2 - 1 mm.






