Device of carbon nanotube growth “CVDomna”
 For today we have already outlined the prospects of the use of carbon nanotubes in composites, nanoelectromechanical systems, sensors and interconnects. The processes of catalytic pyrolysis are the most attractive method for producing carbon nanotubes because of the possibility of its future introduction into electronic technology. Catalytic pyrolysis processes synthesize nanotubes with a predetermined orientation on the other structural components.
For today we have already outlined the prospects of the use of carbon nanotubes in composites, nanoelectromechanical systems, sensors and interconnects. The processes of catalytic pyrolysis are the most attractive method for producing carbon nanotubes because of the possibility of its future introduction into electronic technology. Catalytic pyrolysis processes synthesize nanotubes with a predetermined orientation on the other structural components.
Evaporation techniques (arc discharge, laser ablation) can not grow nanotubes in the predetermined location, but the material obtained by such methods may be applied to a substrate and used for its intended purpose, in this case, the position of the the nanotubes is not controlled. Problems to be solved in the present work have their place in the overall picture of the work on the technology of carbon nanotubes material. Currently, scientists conduct the research on the synthesis of the nanotube material taking into account the role of catalyst during both the synthesis and the nanotubes positioning relatively to other structural components.
CVDomna provides tasks:
1.Catalysts for special purposes
Not every substance can be a catalyst for the synthesis of carbon nanotubes, but their number is limited by the basic requirements:
- the most extensive surface;
- the energy of mixing with carbon 0 <ω <2kT;
- direct eutectic equilibrium with carbon
- good adsorption to carbon;
- not to be passivated in the range of technological conditions.
2.The mechanism of the carbon nanotubes’ growth
In this case, ethanol, entering the chamber, is decomposed to form carbon monoxide. The formed carbon monoxide is adsorbed on the surface by the catalyst particles. On these particles the molecules disintegrate into carbon dissolved in the catalyst and carbon dioxide. The process lasts until the equilibrium state of the system "solution of carbon in the catalyst" - "quasi-liquid adsorbate carbon monoxide” is reached. The catalyst particle, which dissolves the carbon floats in the droplet of its own adsorbed carbon monoxide. On cooling, the carbon dissolved in the particle starts to "go out" of it and the carbon is desorbed from the drop. The drop while moving over the surface leaves the desorbed carbon, in the form of a carbon nanotube.
When carbon is dissolved in the catalyst particle this particle is heated up. If in the end of carbon dedissolution the catalyst particle is not passivated by the carbon film, then nothing prevents us from repeating the process of dissolution- dedissolution with the old carbon nanotube’s growth or initiating a new carbon nanotube’s growth. The only condition is the presence of active carbon-containing molecules in the gas phase. Sequential heating and cooling are not equivalent, because the dissolution includes every atom while dedissolution - every cluster. The study of this process will allow us not only to model the properties of the catalyst for the required tasks, but also to answer the question – “Is there a limit to the length of the carbon nanotube? “.
3.Intercalation of metals into the carbon nanotubes
The use of carbon nanotubes as material carriers guarantees their chemical protection from external environment. We found in our laboratory that the materials involved in the synthesis of carbon nanotubes always fill in the inner cavity of the nanotube.
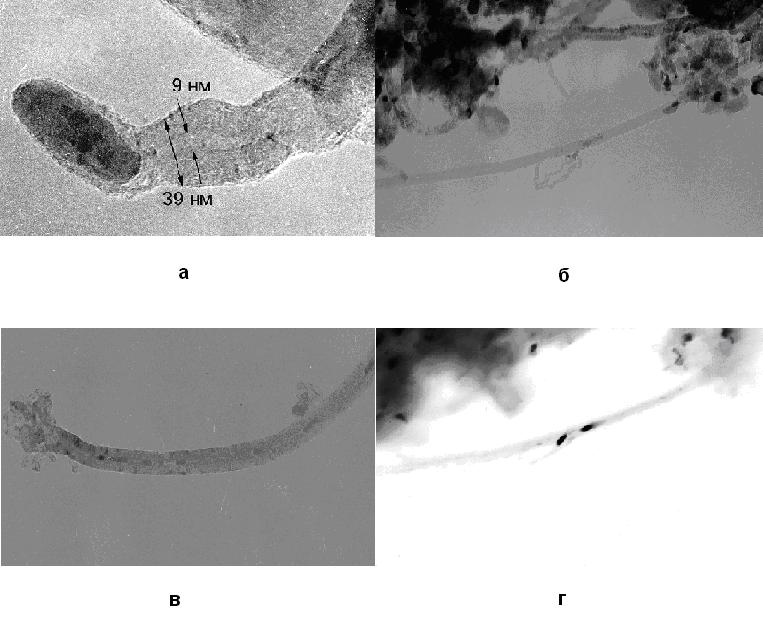
TEM image of the nanotubes, grown in CVDomna